Several French hospitals pause AstraZeneca vaccine campaign over temporary side effects
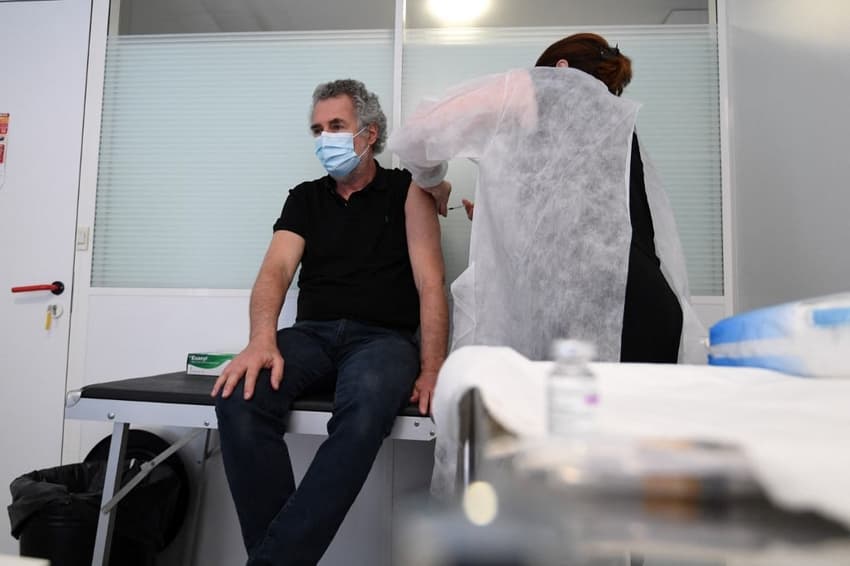
Several French hospitals are pausing or slowing down AstraZeneca vaccination programmes for their staff because severe - albeit temporary - side effects have caused many employees to need sick leave, causing severe logistical problems in already over-stretched services.
Since French health workers started receiving the AstraZeneca vaccine on February 6th, many have reported experiencing side effects including a high fever (40C), fatigue and general flu-like symptoms.
The French health ministry has moved to reassure staff that these effects are temporary and do not mean that the vaccine does not work.
But now some hospitals in France have decided to slow down their vaccination campaigns in order to avoid having too many staff off sick at the same time, while others are pausing them altogether.
Thomas Bourhis, a nurse at CHRU hospital in Brest, Brittany, Vaccin AstraZeneca: pour Thomas Bourhis, infirmier au CHRU de Brest, "les effets secondaires ont été manifestement sous-évalués" pic.twitter.com/YBbyW7rmIx
— BFMTV (@BFMTV) February 12, 2021
Vaccin AstraZeneca: pour Thomas Bourhis, infirmier au CHRU de Brest, "les effets secondaires ont été manifestement sous-évalués" pic.twitter.com/YBbyW7rmIx
— BFMTV (@BFMTV) February 12, 2021
“But it turns out the side effects were underestimated, and we found ourselves with almost a third of health workers in some departments presenting severe symptoms and having to go on sick leave.
“The roll out of this vaccine has therefore been suspended temporarily at the hospital.”
Studies show these secondary effects have nothing to do with the vaccine’s effectiveness, and are not harmful.
"The secondary effects of the Covid-19 vaccines are carefully monitored by ANSM [The French National Drug Safety Agency]. For the AstraZeneca vaccine, it’s important to remember that any side effects remain benign and temporary," the health ministry said in a tweet.
#Vaccination | Les effets indésirables des vaccins contre la #COVID19 sont rigoureusement surveillés par l'@ansm ⤵
✅Pour #AstraZeneca il faut rappeler que d'éventuels effets indésirables restent bénins & temporaires
— Ministère de la Santé et de la Prévention (@Sante_Gouv) February 25, 2021
In a report published on Thursday, the ANSM listed "149 declarations between February 6th and 10th mentioning flu symptoms, often of high intensity". The average age of the health professionals concerned was 34.
To avoid disrupting healthcare services, the ANSM recommended "staggering vaccination in staff".
The Poitiers University Hospital (CHU de Poitiers) has began to do this by avoiding vaccinating entire teams all at once, and at least three hospitals in the west of France have suspended AstraZeneca vaccinations of their staff, according to a report by France Bleu.
A hospital in Saint-Lô in Normandy has also slowed down its vaccinations, while the CHRU de Brest hospital (Finistère) has decided to suspend its vaccination campaign completely, according to Ouest France.
The AstraZeneca vaccine in France is only licenced for under 65s, so until now it has been used almost exclusively on health professionals, while older people visiting vaccine centres get the Pfizer BioNTech or Moderna jabs.
Starting on Thursday, people between the ages of 50 and 64 with underlying health conditions can also begin to receive the AstraZeneca vaccine through their GPs or in the workplace.
The AstraZeneca jab is also expected to be used more widely once France rolls out injections more widely in the community, particularly in pharmacies, since it does not require the super-cold storage of the Pfizer BioNTech vaccine.
Comments (5)
See Also
Since French health workers started receiving the AstraZeneca vaccine on February 6th, many have reported experiencing side effects including a high fever (40C), fatigue and general flu-like symptoms.
The French health ministry has moved to reassure staff that these effects are temporary and do not mean that the vaccine does not work.
But now some hospitals in France have decided to slow down their vaccination campaigns in order to avoid having too many staff off sick at the same time, while others are pausing them altogether.
Thomas Bourhis, a nurse at CHRU hospital in Brest, Brittany, Vaccin AstraZeneca: pour Thomas Bourhis, infirmier au CHRU de Brest, "les effets secondaires ont été manifestement sous-évalués" pic.twitter.com/YBbyW7rmIx
Vaccin AstraZeneca: pour Thomas Bourhis, infirmier au CHRU de Brest, "les effets secondaires ont été manifestement sous-évalués" pic.twitter.com/YBbyW7rmIx
— BFMTV (@BFMTV) February 12, 2021
“But it turns out the side effects were underestimated, and we found ourselves with almost a third of health workers in some departments presenting severe symptoms and having to go on sick leave.
“The roll out of this vaccine has therefore been suspended temporarily at the hospital.”
Studies show these secondary effects have nothing to do with the vaccine’s effectiveness, and are not harmful.
"The secondary effects of the Covid-19 vaccines are carefully monitored by ANSM [The French National Drug Safety Agency]. For the AstraZeneca vaccine, it’s important to remember that any side effects remain benign and temporary," the health ministry said in a tweet.
#Vaccination | Les effets indésirables des vaccins contre la #COVID19 sont rigoureusement surveillés par l'@ansm ⤵
— Ministère de la Santé et de la Prévention (@Sante_Gouv) February 25, 2021
✅Pour #AstraZeneca il faut rappeler que d'éventuels effets indésirables restent bénins & temporaires
In a report published on Thursday, the ANSM listed "149 declarations between February 6th and 10th mentioning flu symptoms, often of high intensity". The average age of the health professionals concerned was 34.
To avoid disrupting healthcare services, the ANSM recommended "staggering vaccination in staff".
The Poitiers University Hospital (CHU de Poitiers) has began to do this by avoiding vaccinating entire teams all at once, and at least three hospitals in the west of France have suspended AstraZeneca vaccinations of their staff, according to a report by France Bleu.
A hospital in Saint-Lô in Normandy has also slowed down its vaccinations, while the CHRU de Brest hospital (Finistère) has decided to suspend its vaccination campaign completely, according to Ouest France.
The AstraZeneca vaccine in France is only licenced for under 65s, so until now it has been used almost exclusively on health professionals, while older people visiting vaccine centres get the Pfizer BioNTech or Moderna jabs.
Starting on Thursday, people between the ages of 50 and 64 with underlying health conditions can also begin to receive the AstraZeneca vaccine through their GPs or in the workplace.
The AstraZeneca jab is also expected to be used more widely once France rolls out injections more widely in the community, particularly in pharmacies, since it does not require the super-cold storage of the Pfizer BioNTech vaccine.
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.