French health regulator recommends AstraZeneca's Covid vaccine for under-65s only
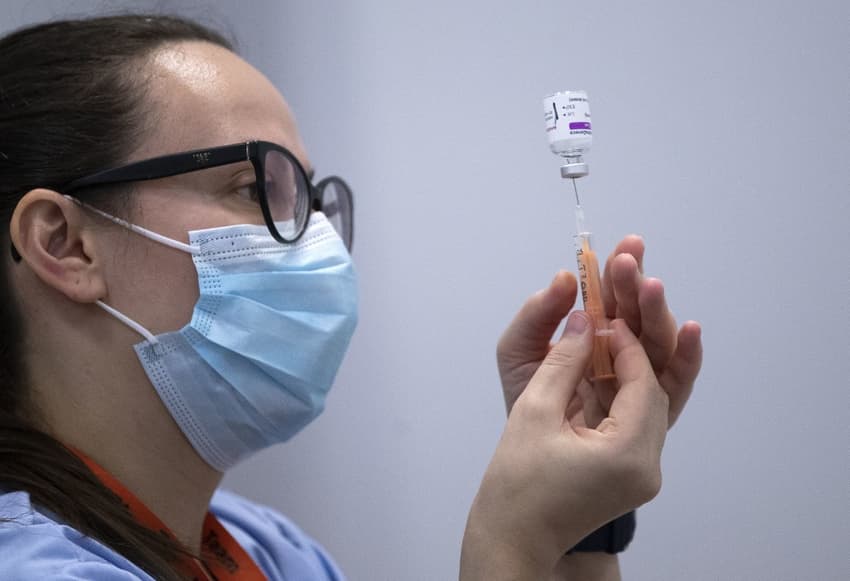
France's top health authority has approved the AstraZeneca vaccine for use on 18 to 65-year-olds only, following the lead of several other European countries who say there is not enough data to show its effectiveness on over 65s.
The European Medicines Agency has licenced the AstraZeneca vaccine for use on all age groups, but health regulators in Germany, Sweden and Austria have cleared it only for 18-65-years old, saying there is not enough data to prove its efficiency for the over 65s.
The French medical body Haute Autorité de santé on Tuesday followed their lead, recommending it only for under 65s.
French President Emmanuel Macron was on Tuesday evening holding an emergency meeting with vaccine producers and laboratories in France.
The object of the meeting was to "take stock of the current state of vaccine production capacity" at the French and European levels and to "call for this capacity to be maximised in the short term" in order to "increase it rapidly and significantly", the president's office said.
The meeting was attended by France's health minister, industry minister and director general of public health, along with the European Commission's health chief Sandra Gallina by video link.
The French pharma giant Sanofi has already announced that it will produce an extra 125 million doses of the Pfizer vaccine, since its own vaccine will not be ready for the market for many months yet.
It comes as the European Commission indicated that it is shifting its early Covid-19 vaccination strategy away from AstraZeneca after the Anglo-Swedish company fell far short in its delivery of doses.
Gallina told MEPs the firm has been able to guarantee just 25 percent of the more than 100 million doses promised and that this was "a real issue" for the EU's 27 countries.
"AstraZeneca was going to be the mass vaccine for quarter one," she said, referring to the first three months of 2021. "The fact that AstraZeneca is not there in the quantities that were stipulated in the contract is quite problematic for all member states."
Gallina added that the Commission was now looking to the vaccines made by BioNTech/Pfizer and Johnson & Johnson to fill the gap.
France had based its vaccine strategy largely on the Pfizer BioNTech and Moderna vaccines for January and February.
Unlike the Pfizer BioNTech vaccine which must be stored at -70C, the AstraZeneca one can be stored at between 4C and 8C and is therefore easier to administer in the community.
The French government has given the go-ahead for pharmacies to begin offering the AstraZeneca, vaccine, with the first doses expected to be available in pharmacies by the third week of February.
French pharmacies already offer vaccines including the flu vaccine, although the Covid jab is expected to be on an appointment basis within the priority group timetables, which place the most vulnerable at the front of the queue.
The French government has been the subject of much criticism for its slow start to the vaccine programme, although this has picked up speed in recent weeks and now 1.4 million people have received the injection.
4 560 personnes ont été vaccinées hier, soit 1 486 493 depuis le début de la campagne vaccinale (données non consolidées) https://t.co/aUNdntMuM1 pic.twitter.com/aIeus9zeXz
— GRZ - CovidTracker (@GuillaumeRozier) February 1, 2021
France has decided not to delay the second dose of the vaccine and is sticking with the manufacturer's recommendation of 3-4 weeks between the first and second dose - a feared shortage of doses lead to some first-dose appointments being cancelled last week.
However even with the increased pace of the vaccination campaign, France is still lagging well behind many other European countries.
La France bon dernier de la classe européenne en part de la population immunisée (personnes ayant reçu deux doses). pic.twitter.com/mRra4Y3Itj
— Vincent Glad (@vincentglad) February 1, 2021
Health minister Olivier Véran has said that everyone who wants the vaccine will have it by the end of August, but supply problems with the AstraZeneca vaccine have lead to France's February target of 4 million people vaccinated being downgraded to "between 2.5 million and 4 million" people.
At present the vaccine programme is France is only to four groups; over 75s, people under 75 with serious health conditions, healthcare workers over 50 or with a health conditions and residents and staff in the country's Ehpad nursing homes.
The next two groups - 65-74-year-olds and all healthcare workers - are expected in February but no date has yet been set for this.
Anyone who is in a priority group can book an appointment direct.
READ ALSO How to book an appointment for the Covid vaccine in France
Comments (3)
See Also
The European Medicines Agency has licenced the AstraZeneca vaccine for use on all age groups, but health regulators in Germany, Sweden and Austria have cleared it only for 18-65-years old, saying there is not enough data to prove its efficiency for the over 65s.
The French medical body Haute Autorité de santé on Tuesday followed their lead, recommending it only for under 65s.
French President Emmanuel Macron was on Tuesday evening holding an emergency meeting with vaccine producers and laboratories in France.
The object of the meeting was to "take stock of the current state of vaccine production capacity" at the French and European levels and to "call for this capacity to be maximised in the short term" in order to "increase it rapidly and significantly", the president's office said.
The meeting was attended by France's health minister, industry minister and director general of public health, along with the European Commission's health chief Sandra Gallina by video link.
The French pharma giant Sanofi has already announced that it will produce an extra 125 million doses of the Pfizer vaccine, since its own vaccine will not be ready for the market for many months yet.
It comes as the European Commission indicated that it is shifting its early Covid-19 vaccination strategy away from AstraZeneca after the Anglo-Swedish company fell far short in its delivery of doses.
Gallina told MEPs the firm has been able to guarantee just 25 percent of the more than 100 million doses promised and that this was "a real issue" for the EU's 27 countries.
"AstraZeneca was going to be the mass vaccine for quarter one," she said, referring to the first three months of 2021. "The fact that AstraZeneca is not there in the quantities that were stipulated in the contract is quite problematic for all member states."
Gallina added that the Commission was now looking to the vaccines made by BioNTech/Pfizer and Johnson & Johnson to fill the gap.
France had based its vaccine strategy largely on the Pfizer BioNTech and Moderna vaccines for January and February.
Unlike the Pfizer BioNTech vaccine which must be stored at -70C, the AstraZeneca one can be stored at between 4C and 8C and is therefore easier to administer in the community.
The French government has given the go-ahead for pharmacies to begin offering the AstraZeneca, vaccine, with the first doses expected to be available in pharmacies by the third week of February.
French pharmacies already offer vaccines including the flu vaccine, although the Covid jab is expected to be on an appointment basis within the priority group timetables, which place the most vulnerable at the front of the queue.
The French government has been the subject of much criticism for its slow start to the vaccine programme, although this has picked up speed in recent weeks and now 1.4 million people have received the injection.
4 560 personnes ont été vaccinées hier, soit 1 486 493 depuis le début de la campagne vaccinale (données non consolidées) https://t.co/aUNdntMuM1 pic.twitter.com/aIeus9zeXz
— GRZ - CovidTracker (@GuillaumeRozier) February 1, 2021
France has decided not to delay the second dose of the vaccine and is sticking with the manufacturer's recommendation of 3-4 weeks between the first and second dose - a feared shortage of doses lead to some first-dose appointments being cancelled last week.
However even with the increased pace of the vaccination campaign, France is still lagging well behind many other European countries.
La France bon dernier de la classe européenne en part de la population immunisée (personnes ayant reçu deux doses). pic.twitter.com/mRra4Y3Itj
— Vincent Glad (@vincentglad) February 1, 2021
Health minister Olivier Véran has said that everyone who wants the vaccine will have it by the end of August, but supply problems with the AstraZeneca vaccine have lead to France's February target of 4 million people vaccinated being downgraded to "between 2.5 million and 4 million" people.
At present the vaccine programme is France is only to four groups; over 75s, people under 75 with serious health conditions, healthcare workers over 50 or with a health conditions and residents and staff in the country's Ehpad nursing homes.
The next two groups - 65-74-year-olds and all healthcare workers - are expected in February but no date has yet been set for this.
Anyone who is in a priority group can book an appointment direct.
READ ALSO How to book an appointment for the Covid vaccine in France
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.