France bans breast implants from new firm
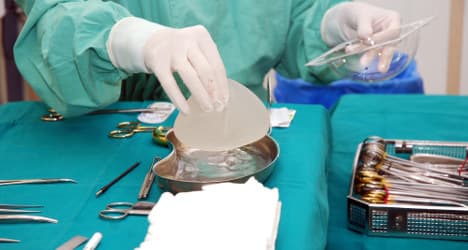
France, still shaken from a faulty breast implant scandal that saw thousands of women suffering ruptures, has banned implants made by a South Korean company after the firm’s products failed to meet safety standards.
The national drug and health safety agency, ANSM, banned Hans Biomed Corporation from selling its products after the biotechnology firm refused to let its inspectors visit its production site, as it said its factory was being rebuilt.
ANSM said Hans Biomed implants were available in Europe under three brand names - Bellagel, M-Implants and Natureshape - but that these were not all available in France.
The agency was able to examine the latter two brands and said that “the quality of these breast implants highly questionable”.
It did not elaborate on what the problems were, nor did it say how many, if any, women had had these faulty implants put in place.
No one was immediately available at ANSM to provide further details.
When ANSM revoked Hans Biomed’s authorisation in February this year it informed the European Union authorities of its move, who in turn informed national authorities across Europe.
Medical devices such as breast implants come under national and not EU responsibility, so it would be up to individual countries to decide whether to follow France’s lead.
No-one was immediately available in Hans Biomed’s headquarters in Seoul to comment on the ban.
The news of the ban came in a major report published on Tuesday by ANSM inspectors who had visited all 11 sites in France where implants are made as well as the various distribution centres.
One French firm, Cereplas, was ordered to suspend its implant sales while it raised its standards to the legal norms but ANSM said its products did not pose a threat to health.
The inspections were carried out in the wake of a scandal in which 300,000 women in 65 countries are believed to have received faulty implants made from industrial-grade silicone by French firm PIP.
More than 7,500 women have reported ruptures and in France alone more than 15,000 have had the PIP implants replaced.
The ANSM report painted a worrying picture for the 340,000 women in France who have had their breasts enlarged.
It said that that 2,169 women - aside from those who got faulty PIP implants - had since 2010 reported that their implants had ruptured.
The report said the breast implants on offer by various companies lasted on average 7.6 years, and not the ten years promised by their manufacturers.
It also said that women who had implants suffered more frequently from lymphatic cancer than the general population. by Rory Mulholland
Comments
See Also
The national drug and health safety agency, ANSM, banned Hans Biomed Corporation from selling its products after the biotechnology firm refused to let its inspectors visit its production site, as it said its factory was being rebuilt.
ANSM said Hans Biomed implants were available in Europe under three brand names - Bellagel, M-Implants and Natureshape - but that these were not all available in France.
The agency was able to examine the latter two brands and said that “the quality of these breast implants highly questionable”.
It did not elaborate on what the problems were, nor did it say how many, if any, women had had these faulty implants put in place.
No one was immediately available at ANSM to provide further details.
When ANSM revoked Hans Biomed’s authorisation in February this year it informed the European Union authorities of its move, who in turn informed national authorities across Europe.
Medical devices such as breast implants come under national and not EU responsibility, so it would be up to individual countries to decide whether to follow France’s lead.
No-one was immediately available in Hans Biomed’s headquarters in Seoul to comment on the ban.
The news of the ban came in a major report published on Tuesday by ANSM inspectors who had visited all 11 sites in France where implants are made as well as the various distribution centres.
One French firm, Cereplas, was ordered to suspend its implant sales while it raised its standards to the legal norms but ANSM said its products did not pose a threat to health.
The inspections were carried out in the wake of a scandal in which 300,000 women in 65 countries are believed to have received faulty implants made from industrial-grade silicone by French firm PIP.
More than 7,500 women have reported ruptures and in France alone more than 15,000 have had the PIP implants replaced.
The ANSM report painted a worrying picture for the 340,000 women in France who have had their breasts enlarged.
It said that that 2,169 women - aside from those who got faulty PIP implants - had since 2010 reported that their implants had ruptured.
The report said the breast implants on offer by various companies lasted on average 7.6 years, and not the ten years promised by their manufacturers.
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.